English
Views: 0 Author: Site Editor Publish Time: 2025-09-26 Origin: Site
ABSTRACT
Oxygen scavenging serves as a critical strategy in pharmaceutical packaging, with the primary goal of preventing drugs from undergoing oxidation and degradation. By integrating advanced active materials science technology into active packaging solutions, which actively eliminates oxygen from the packaging headspace, pharmaceutical companies can guarantee the stability and effectiveness of their products throughout the entire shelf-life. Sophisticated oxygen scavenging technology can be seamlessly incorporated into existing packaging formats and production processes in the form of films (such as heat-staked films and blown films), minimizing disruptions to manufacturing workflows.
INTRODUCTION
Oxygen scavenging technology plays a vital role in enhancing the stability and extending the shelf-life of various pharmaceutical and food products when applied in their packaging. Even trace amounts of oxygen can trigger the oxidation and subsequent degradation of active pharmaceutical ingredients (APIs), thereby compromising the efficacy, safety, and appearance of the drugs. The oxidation process may lead to the formation of harmful by-products, reduce the potency of APIs, and alter physical properties like color and solubility. As a result, controlling and minimizing oxygen levels within pharmaceutical packaging is of utmost importance for maintaining drug quality.
The application of oxygen scavenging technology is particularly crucial for pharmaceuticals that are highly sensitive to oxidation, including certain antibiotics, vitamins, and biologics. Notably, the use of oxygen scavengers can reduce the requirement for preservatives and antioxidants in drug formulations, potentially lowering the risk of adverse reactions.
A variety of strategies can be employed to address the risks posed by oxygen in the packaging headspace. Common methods used to reduce oxygen levels in packaging are as follows:
Barrier Materials: Packaging materials with high oxygen barrier properties can be utilized to prevent external oxygen from entering the package.
Modified Atmosphere Packaging (MAP): In MAP, the internal atmosphere of the package is adjusted by replacing oxygen with inert gases (e.g., nitrogen, carbon dioxide).
Chemical Scavengers: These include organic scavengers (ascorbic acid, ascorbic acid salts, isoascorbic acid, tocopherol, hydroquinone, catechol, rongalite, sorbose, lignin, gallic acid, lecithin, rosemary extracts, polyunsaturated fatty acids, threonine), metallic scavengers (iron powder, activated iron, ferrous oxide, iron salts, iron nanoparticles, Co (II), Zn, platinum, palladium), inorganic (non-metallic) scavengers (sulfite, thiosulfate, dithionite, hydrogen sulfite, titanium dioxide), and polymer-based scavengers (redox resins, polymer-metal complexes).
Enzymatic Scavengers: The most prominent ones are glucose oxidase, laccase, and ethanol oxidase. Enzymes like glucose oxidase catalyze the conversion of glucose into gluconic acid and hydrogen peroxide; the latter then reacts with oxygen, reducing oxygen levels.
The first two strategies (barrier materials and modified atmosphere packaging) can be used to protect unit-dose (individual) pharmaceutical packaging. However, they are inefficient for multi-dose packaging. This is because external oxygen can enter the package when users repeatedly open and close it. A widely adopted solution is the use of oxygen-scavenging materials, which can be integrated into packaging systems to protect sensitive products by actively removing oxygen from the surrounding environment. These scavengers can be incorporated into various parts of the packaging, such as bottle closures, blister packs, or sachets, and function through chemical reactions that absorb or adsorb oxygen. Commonly used oxygen-scavenging materials include iron powder and ascorbic acid, and the choice between them depends on the specific requirements of the pharmaceutical product and the packaging design.
Iron-based scavengers operate through an oxidation process, where iron reacts with oxygen to form iron oxide, effectively reducing the oxygen concentration. Ascorbic acid, another widely used scavenger, reacts with oxygen to form dehydroascorbic acid. When metallic systems are not allowed, non-metallic scavengers can be a viable alternative, among which sulfites are highly effective oxidative compounds.
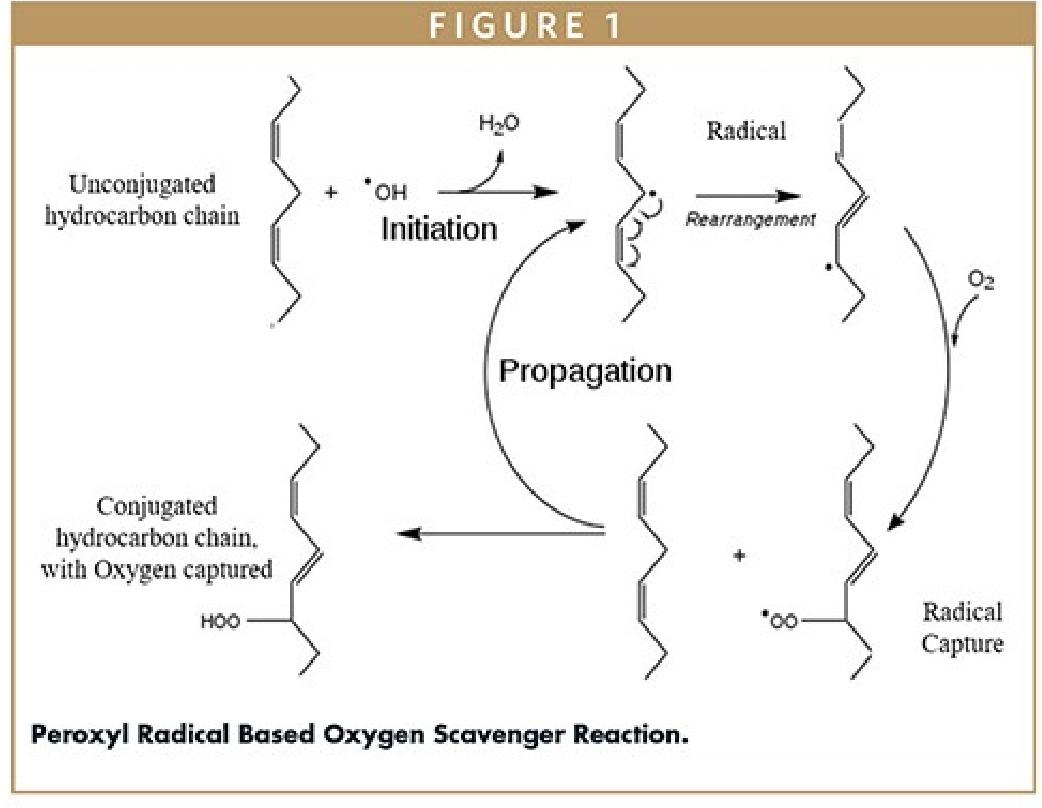
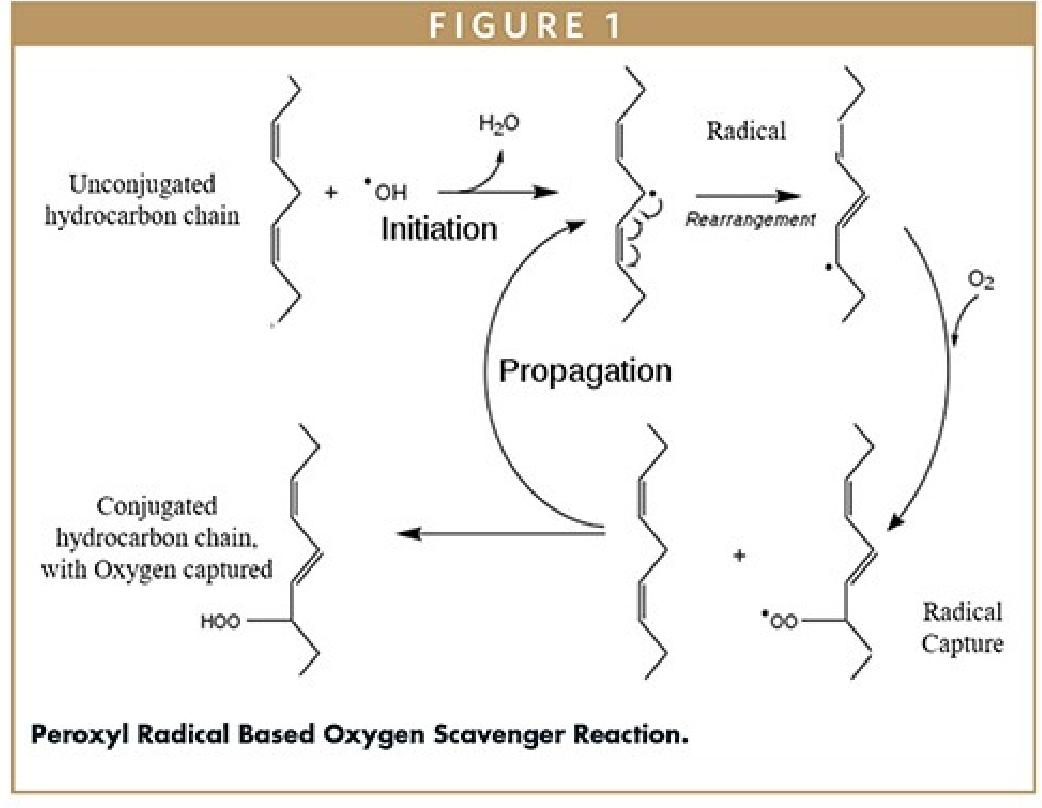
An alternative technology that does not require water, generates peroxyl radicals, and ultimately acts as an oxygen scavenger has also attracted attention. In this technology, peroxyl radicals are produced through the direct interaction between oxygen and alkyl radicals. The formation of peroxyl radicals is limited by the amount of oxygen available in the headspace of a sealed package. An unconjugated chain reacts with the peroxyl radicals to capture oxygen, and then the chemical bonds rearrange to form an oxygen-bound conjugated chain (Figure 1 illustrates this reaction process). Depending on the length of the chain, multiple oxygen molecules can be captured. Once oxygen is bound to the hydrocarbon chain, the resulting compound becomes very stable. These types of scavengers can be modified with photoinitiators to prevent premature oxidation of the scavenger during processing. Enzyme-based oxidation is another approach to regulating oxygen concentration in food packaging. Enzymatic scavengers use enzymes to catalyze reactions that convert oxygen into less reactive substances. The selection of these materials typically considers their compatibility with food and pharmaceutical products, the desired rate of oxygen removal, and regulatory requirements. Some of them can start scavenging oxygen immediately upon exposure to oxygen or air under ambient humidity and temperature conditions. On the other hand, some oxygen scavengers require an activation mechanism (such as water vapor, ultraviolet light, or magnetic fields) to control the initiation of the reaction. This ensures that the oxygen-scavenging system remains stable before being used with drugs and avoids premature activation.
The following section explores how chemical oxygen scavengers can be applied using a novel active polymer platform for oxygen scavenging. This approach can significantly reduce the amount of oxygen in the packaging headspace that could otherwise cause destructive reactions and ultimately lead to a decline in drug functionality.
METHODS


Pioneered by Aptar CSP Technologies, the 3-Phase Activ-Polymer™ platform technology uses active materials as fillers in composite materials (Figure 2). This proprietary technology is delivered in a unique formulation, which consists of a base polymer (the majority component, providing structural support), a channeling agent, and active particles. In this study, the polymer was used in the form of an active film.
As the first step, six types of oxygen scavengers (five raw active chemical oxygen scavengers and one enzymatic scavenger) were engineered for incorporation into a polymer matrix (Figure 3). Among the five chemical scavengers, three use humidity as a trigger for oxygen scavenging, and two work under dry conditions. The incorporation of oxygen-scavenging systems into the polymer matrix may affect the functional properties of the plastic, including tensile strength, elongation, gas barrier properties, thermal properties, and optical properties. Therefore, during the development of the oxygen-scavenging films, considerations were given to the chemical and physical compatibility with the scavenger, as well as the stability of the active phase during the manufacturing processes. Additionally, good dispersion of the scavenging agent was achieved through sufficient shear force during the extrusion process, which is evident from the uniform dispersion of the active materials in the polymer matrix of Oxygen-Scavenging Film #2 (Figure 2).
Twelve glass bottles (with a volume of 120 mL) were cleaned and equipped with oxydots. A 1×1 square inch piece of one of the six different active films was placed in each bottle (two bottles for each type of film) (Figure 3). For the wet oxygen-scavenging system, a 1×1 square inch filter paper impregnated with a specific volume of water (as shown in Table 1) was also added to the bottle.
The bottles were then crimp-sealed, and an initial oxygen reading was taken using the OxySense system (Gen III, 5000 series) before being stored in an environmental chamber set at 25°C and 60% relative humidity (RH). Using the OxySense system, two samples of each of the six types of films (Figure 3) were tested daily for 200 days.
The OxySense system employs a luminescence method to calculate the amount of oxygen in a closed system. Oxygen Absorption Rate (OAR) tests were conducted for both wet and dry oxygen-scavenging films (Table 1). After collecting the OAR data, the percentage change in oxygen within each bottle was calculated by subtracting the final oxygen percentage from the initial oxygen percentage in the bottle. The amount of oxygen present in the bottle was calculated by multiplying the volume of the bottle (120 mL) by the percentage change in oxygen. Subsequently, the OAR was calculated by dividing the amount of oxygen present by the elapsed time.
(Figure 2: The left image is a Scanning Electron Microscope (SEM) image of Oxygen-Scavenging Film #2, showing the dispersion of active particles in the polymer matrix under a certain magnification, with a scale bar marked as 400 μm; the right image is a locally magnified SEM image, providing a clearer view of the distribution details of the active particles, also with a scale bar of 400 μm)

RESULTS & DISCUSSION
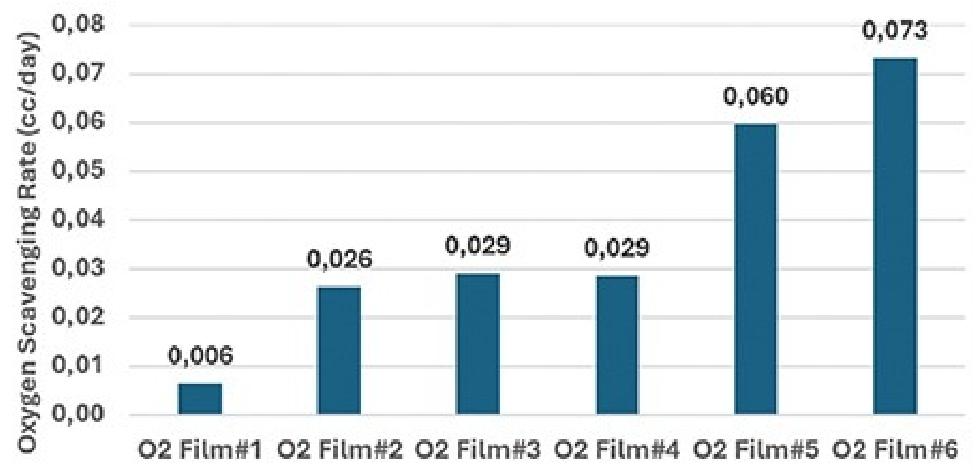
Figure 4 shows the OAR of the six oxygen-scavenging films over the entire 200-day period.
O2 Film #5 and O2 Film #6, which can react with oxygen in the absence of humidity, exhibited the highest OAR values among the films that could be used for 200 days. O2 Film #5 had an OAR of 0.060 cc/day, while O2 Film #6 showed the highest OAR value of 0.073 cc/day, which was 0.013 cc/day higher than that of O2 Film #5.
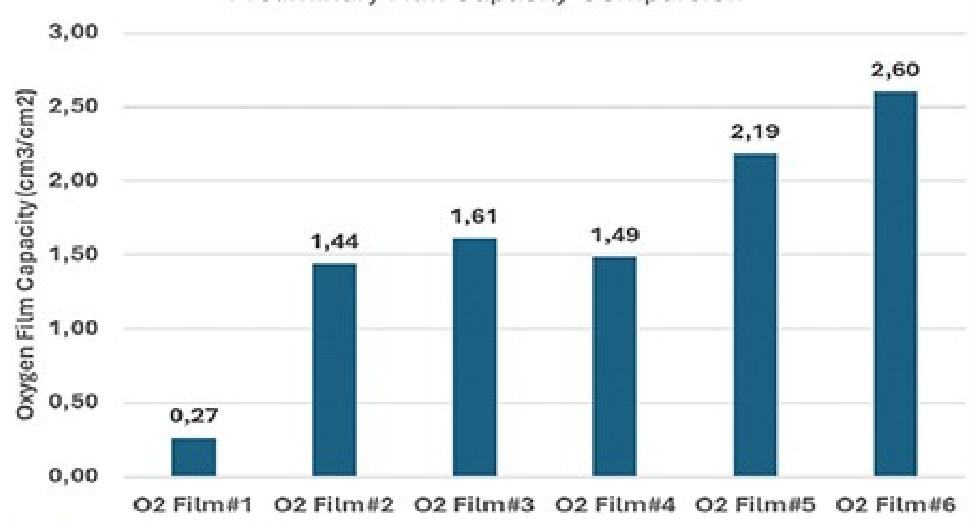
After collecting all the test data, the oxygen-scavenging capacity of the films was calculated. First, the amount of oxygen remaining in the bottle was calculated by multiplying the bottle volume (120 mL) by the minimum oxygen reading in the dataset. Next, the amount of oxygen scavenged was determined by subtracting the remaining oxygen from the original amount of oxygen in the bottle (the original oxygen content was assumed to be 21%). Finally, the capacity was calculated by dividing the amount of oxygen scavenged by the surface area of the sample (1 square inch, equivalent to 6.45 cm²). Figure 5 shows the capacities of the tested films.
The calculated capacity of O2 Film #5 was 2.19 cm³/cm², which was higher than that of most other tested films. The capacity of O2 Film #6 was the highest, reaching 2.60 cm³/cm², which was 16% higher than that of O2 Film #5.
CONCLUSION
Oxygen scavengers will continue to play a crucial role in pharmaceutical packaging by protecting drugs from oxidative degradation. As pharmaceutical manufacturers strive to optimize costs and maintain drug potency, these scavengers help extend the shelf-life of drugs and prevent adverse effects caused by oxygen exposure. Incorporating scavengers directly into packaging enables pharmaceutical developers to reduce the risk of drug degradation, enhance drug stability, and meet regulatory requirements without the need to reformulate their drugs.
The novel 3-Phase Activ-Polymer™ oxygen-scavenging films developed by Aptar CSP Technologies can actively remove oxygen from the packaging headspace. For drugs susceptible to oxidative degradation, these films can significantly extend their shelf-life and ensure that the drugs remain effective throughout their intended period of use.
(Note: All images in this document are schematic descriptions. Actual images can be obtained by searching for similar oxygen scavenging reaction process diagrams, film SEM microstructural diagrams, oxygen absorption rate trend diagrams, and oxygen scavenging capacity comparison bar charts from scientific image databases or relevant technical literature to ensure that the image content is consistent with the technical theme.)
